
Image by The Whisky Ardvark
Many spirit drinkers will have a basic understanding of what happens during a distillation run in a copper pot still and are familiar with the concept of heads, hearts, and tails. But what exactly happens when a spirit like whisky is distilled, and why is the distillation divided into these separate cuts?
We decided to take a look at the compounds produced during distillation and the relevant temperatures applied. Please note that we are not experts in chemistry, and this article is written only as a guideline for those who wish to understand the basics applied to distilling for an easy approach. Please also note that we have not separated a wash run (resulting in ABV ~20%) and a spirited run (ABV ~ mid-70 %).
Since we are The Whisky Ardvark, we have chosen to display the aardvark as our animal of choice to help with the visualising of a distillation run.
We understand that a lot of this sounds terrifying, but the people using stills and distilling spirits professionally know what they are doing. So don't let this article scare you from enjoying your whisky.
From Heads to Tails
A distillation run is usually divided into 4 different cuts - Foreshots, Heads, Hearts, and Tails. Separating the sections is done due to changing temperatures that extract different compounds during the run. Needless to say, the desired result is the purest potable alcohol without chemicals that can be dangerous and harmful to the human body.
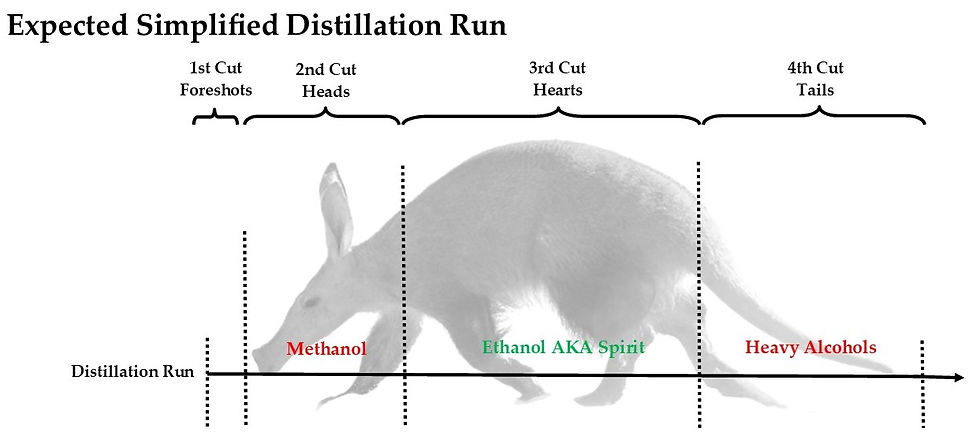
Image by The Whisky Ardvark
Foreshots & Heads
Many times, the unmentioned part of the distillation is the Foreshots, which can also be classified as part of the Heads. We have separated the two from each other because the Foreshots have the most volatile and dangerous components and, unlike the main Heads, can not be redistilled.
Foreshots are the home of methanol - a lower boiling point alcohol that can cause blindness and, in the worst-case scenario, death. Methanol has a boiling point of 64.96 degrees Celsius, which is lower than the desired Ethanol. When the still and the mash/ spirit inside it heats up, Methanol is the first to make an appearance among other chemicals.
Other compounds that can be found in the Heads include:
Acetaldehyde - With a boiling point of 20.8 C, Acetaldehyde is produced by the oxidation of ethanol. It is a natural by-product of plant metabolism, but if consumed, it can cause a fast heartbeat, a headache, an upset stomach, problems with brain activity, and memory impairment. Acetaldehyde is a major contributor to the severity of a hangover.
Acetone - Acetone is the simplest form of ketone, with a boiling point of 56.2 C. It is the active ingredient in nail polish removers, paint thinners, and cleaning solvents. If ingested, it can cause headache, movement problems, tiredness, slurred speech, nausea, vomiting, and fitting. Yeah, that's not what you want to have in your drink.
Ethyl Acetate - Ethyl Acetate is a type of ester and has a boiling point of 77.1 C. It has a relatively pleasant smell and taste and is used as a food flavour enhancer in small amounts. It is also used to remove caffeine from tea and coffee. As a downside, Ethyl Acetate is highly flammable and, if inhaled or digested in large amounts, can cause serious damage to the inner organs.
Esters - Esters are found in many fruits and contribute to the 'fruity' aroma of both produce and alcohol. Some esters linger in the still and are desirable taste contributors to the spirit produced. But! Low-molecular-weight esters are highly flammable and, if ignited, release many toxic gases. Fatty acid ethyl esters have been implicated as important mediators of ethanol-induced cytotoxicity, organ damage, and disease.
The Hearts
AKA the Best Part of The Chicken (or an aardvark), since the tastiest part of the distillation lies in the 'middle'. Ethanol has a boiling point of 78.37 C, and although called potable alcohol, it is still a foreign poison to our bodies if consumed recklessly. Ethanol has an effect on the human body just like any other recreational drug, with the ability to impair the nervous system and affect mood and behaviour. It is also highly flammable.
Since Methanol and Ethanol are famously hard to separate from each other even by distillation, the Hearts will have traces of Methanol in them - but the amount is deemed safe for human consumption (although highly monitored). Separating Ethanol from water is a lot easier since water's boiling point is 100 C.
Tails
The Tails are the last piece of the puzzle in distillation. With the rising temperature inside the still, the heavy-weight alcohols make an appearance. Even though many of the compounds produced at these levels of heat are dangerous or even toxic to consume or inhale (especially in large amounts), they offer complexity to the spirit produced if applied correctly.
A distillation run temperature is normally kept under 96 C. Many of the following chemicals will have a boiling point higher than that, but small amounts of these compounds will start to heat up and show up in the distillate before reaching its boiling point. Also, with the boiling point of water at 100 C, keeping the temperature under 100 C concentrates the distillate further.
When the distillation run reaches the point of Tails, the smell of the distillate changes and can be smelled throughout the distillery. Many of these undesired compounds have an easily recognisable smell characteristic - some of them extremely unpleasant.
1-Propanol - Even though 1-Propanol is many times compared to Ethanol with its ability to affect the central nervous system, it is more neurotoxic. Sometimes referred to as "Fusel Oil" by distillers, Propanol appears in small amounts during the fermentation process, but as a distillate is used by the pharmaceutical industry as a solvent. The boiling point of 1-Propanol is 97 C. Its other uses include as a component in printing ink, cosmetics and lotions, and window cleaners.
Butanol Alcohol - Butanol is a common by-product of fermented sugars and, therefore, is commonly found in small amounts of beer and other fermented products. Butanol has a boiling point of 118 C and can cause toxic vapours. In extreme conditions, the vaporised Butanol can suppress the nervous system and even cause death. Butanol has a banana-like solvent character.
Amyl Alcohol, AKA Isobutyl Carbinol Ketone (MIBK) -With a boiling point of 131.6 C, Amyl has a sharp and strong burnt smell. It is classified as unsuitable for human consumption. It can cause kidney and liver damage, and if inhaled, vapours can cause nausea, light-headedness, and even loss of consciousness. Amyl Alcohol has its uses as a solvent, synthetic flavourings, and other chemicals.
Acetic Acid - Acetic Acid is known for giving vinegar its pungent smell and taste. It is a naturally occurring acid that forms during fermentation, but as a distillate, can be extremely corrosive to tissues. Acetic Acids' boiling point is 118.1 C.
Furfural - This devious aromatic aldehyde has a boiling point of 161.7 C but tends to make an appearance in much lower temperatures during distillation. Furfural has a distinctive almond smell and turns slightly yellow when it comes in contact with oxygen. It is an irritant that targets the skin, mucous membrane, and respiratory system.
Did you know that Spirit Safe is made of brass since brass is unable to create a spark? This is extremely important when dealing with volatile and flammable compounds.

Image by islay.org.uk
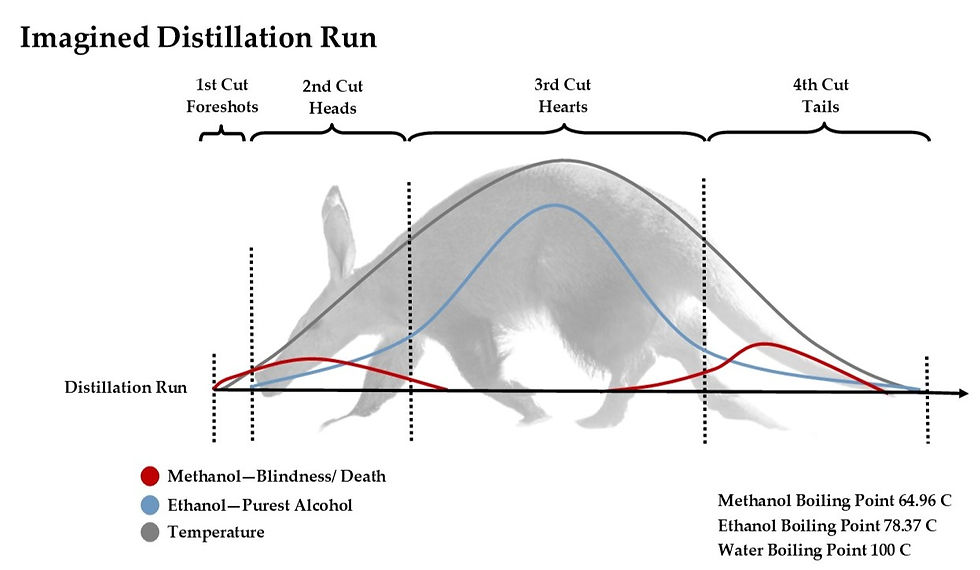
Image by The Whisky Ardvark
With the use of terms like Heads and Tails, it's easy to imagine the distillation run in the shape of an animal. Starting from the Heads, rising to the Hearts, and finishing with the Tails, right? Well, yes and no. For a long time, we had the impression that the Heads and Tails were created by the rise and the drop of the temperature inside the still - resulting in dangerous chemicals. When, in fact, the Tails are full of heavy-weight alcohols caused by the rise in temperature.
In reality, the 'animal in the still' has more room around it - cased between low (<64 C) and high temperatures (>93-96 C).
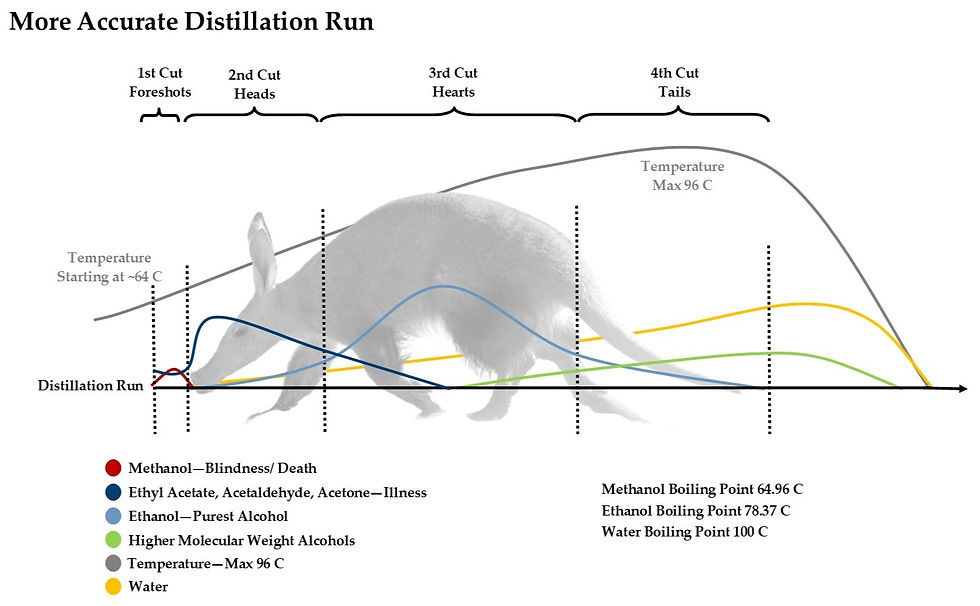

Images by The Whisky Ardvark
Water is also an important compound in distillation. The percentage of water in a still rises when other chemicals make their exit through the top of the still as vapours.
It is also important to point out that not all distilleries cut the Hearts at the same points. For example, Macallan is known for taking only a relatively small cut as Hearts, resulting in a lighter-style distillate with a 'purer' ethanol content, but ultimately, it also restricts the flavour profile.
An important factor to the resulting spirit is also the size of the still used. We have used the term 'heavier compounds never make it to the top' multiple times when describing the giraffe-size stills of Glenmorangie. The taller the still, the less heavy-weight alcohols can climb up the neck of the stills, resulting in a lighter spirit. In reverse, the small stills at Mortlach can produce whisky with a heavier, meaty character than some of its counterparts.
We find that the phrase 'It's not the size of the still, but how you use it' holds when deciding the cutting points of the distillation run, but unlike some men like to think - the size of the still does matter.
Stay tuned to The Whisky Ardvark with #whiskyardvark #thewhiskyardvark, and let us know what you think!

.png)
Comments